
KOSHINÉ 826 Anti-Hair Loss Solution
15 years of R&D by a listed pharmaceutical company;
Patented core ingredient of KOSHINÉ 826 has been clinically proven to be safe and effective for androgenic alopecia.
Features:
Oil control on scalp in 3 days, Hair loss reduction in 1-2 weeks, and New hair growth in 4-6 weeks.
Suitable for:
People with the demand for oil control, anti-hair loss and new hair growth
KOSHINÉ 826’s patented core ingredient 826 has demonstrated excellent safety and efficacy trend in clinical trials and can effectively mitigate the problems of “oily hair, hair loss and baldness”. Regular use can finetune the growth cycle of hair follicles, promote new hair growth, strengthen the hair roots and achieve a thicker and healthy hair.
Product efficacy

KOSHINÉ 826’s patented core ingredient 826 is an androgen receptor antagonist. It competes with dihydrotestosterone (DHT) for binding to androgen receptors in target tissues, blocking the signal transduction meditated by androgen molecules, effectively lowering the effects of androgen on hair follicles and restoring the respective cells to health. It also reduces scalp sebaceous hyperplasia and oil secretion, achieving the effects of oil control, anti-hair loss, and new hair growth. Regular use can extend the growth cycle of hair follicles and build a healthy hair.
826 INCI Name:
Methylpyridinyl Fluoromethoxybenzonitrile Dimethyloxothiooximidazolidine
Oil Control
Anti-hair loss
New hair growth
Mechanism of Action
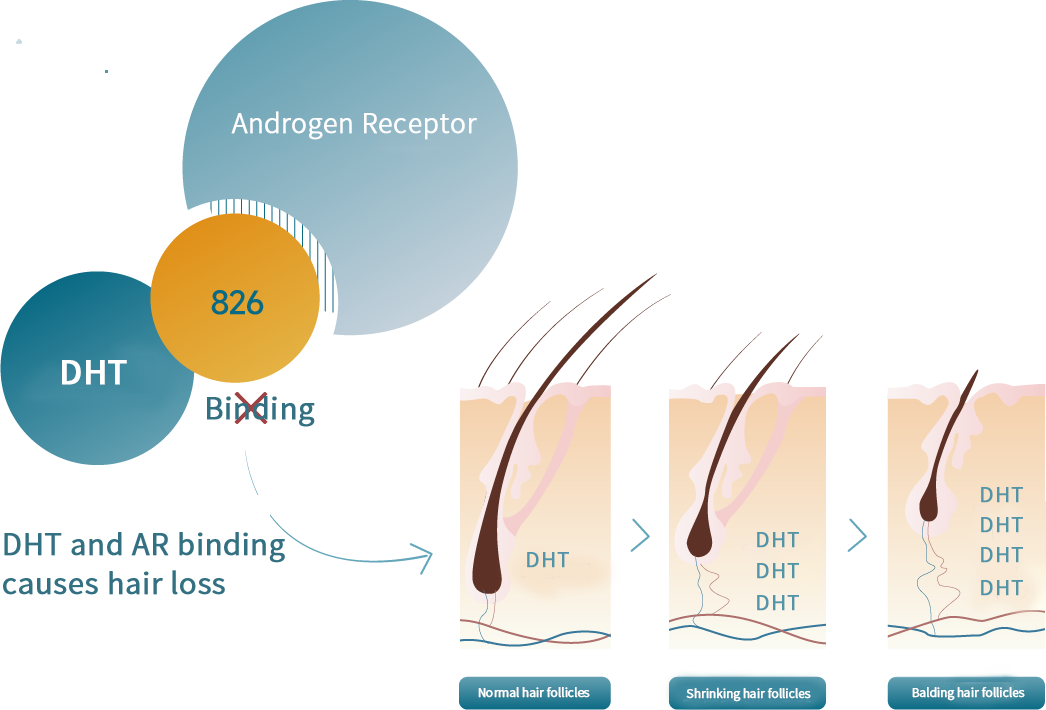
Studies have shown that enhanced expression of androgen receptor genes in the hair follicles of androgenetic alopecia increases the sensitivity of androgen receptors, promotes the binding of DHT to them, and initiates a chain reaction. The hair follicles undergo miniaturization, resulting in gradually hair thinning and the shortening of the hair growth cycle. Over time, the original thick and dark hair gradually transitions to finer and lighter vellus hair. As follicles shrink and eventually disappear, the vellus hair sheds, leading to baldness. This process also results in scalp sebaceous hyperplasia and increases oil secretion.
Test data
EXPERIMENTAL DATA
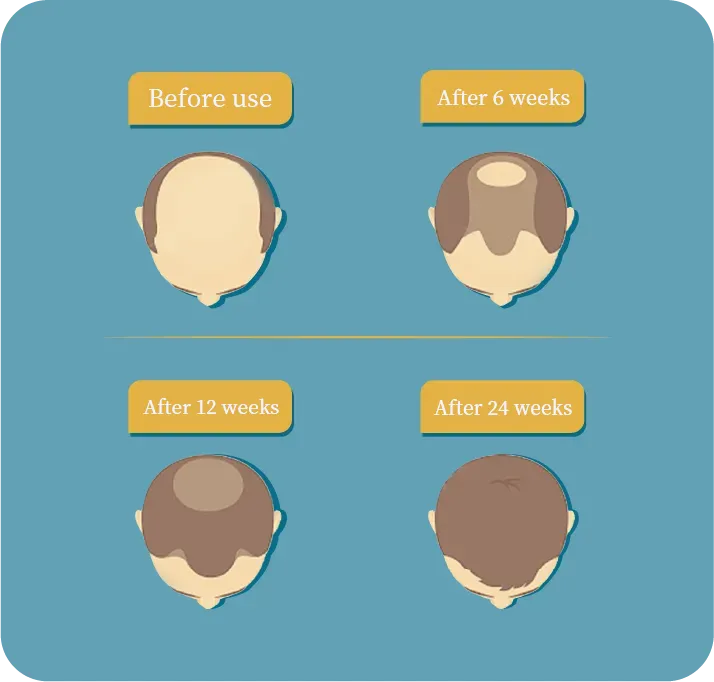
According to our clinical study reports, 826 has demonstrated good efficacy and safety profile. Compared to baseline, the target area hair counts (TAHC) of the 0.5% BID (twice daily) trial group increases 22.73 hair counts per cm^2 on average after 24th weeks of treatment. Some trial users experienced new hair growth as early as in the 6th week.
Data source: Phase II clinical study report of KX0826-CN-1002



